Integro Theranostics announces positive Phase 1b clinical trial data, advancing CXL into Phase II Study
Lung cancer surgery study results for proprietary tumor-targeting agent, CXL (formerly known as LS301), shows promising preliminary safety and efficacy for IMI.
Our mission is to illuminate cancer with innovation and augmented intelligence by merging science and technology, making hope visible for cancer patients undergoing surgery.”
SAINT LOUIS, MO, UNITED STATES, November 17, 2025 /EINPresswire.com/ -- Positive results from the Phase 1b clinical trial of cronexitide lanocianine (CXL), formerly known as LS301, Integro Theranostics’ proprietary tumor-targeting agent for Intraoperative Molecular Imaging (IMI), support advancement into a Phase II lung cancer study planned for late 2025.— Scott Clover, CEO
Updated clinical study data from “A Single Dose, Open-Label, Dose-escalation Study of the Safety and Imaging Characteristics of LS301-IT for Intraoperative Imaging of Lung Cancer,” was presented at the Precision Surgery Intraoperative Molecular Imaging Conference (4th Clinical Trials Update) on Nov. 14, 2025, in Philadelphia. The lead investigator was Sunil Singhal, MD from the University of Pennsylvania.
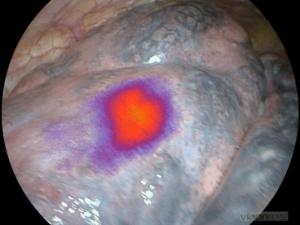
The clinical trial evaluated CXL which was developed by Dr. Samuel Achilefu during his tenure at Washington University School of Medicine in St. Louis, Mo. The findings from this Phase 1b lung cancer surgery clinical trial demonstrated:
• Promising findings for CXL as a potentially important aid for lung cancer surgeons
• Identification of previously undetected or unseen cancer lesions (“Clinically Significant Events”) in 48% of the 23 patients in the study who underwent lung cancer surgery
• Potential guidance for surgeons to ensure adequate margins for lung cancer resection
• CXL was found to be well tolerated with no reported serious adverse events for all patients in the study
• Impactful data and learnings from a diverse patient cohort, with more than one-third of participants coming from underrepresented populations
“Our mission is to illuminate cancer with innovation and augmented intelligence by merging science and technology, making hope visible for cancer patients undergoing surgery,” said Scott Glover, chief executive officer. “The results from our Phase 1b lung study demonstrate the potential to improve intraoperative cancer visualization.”
The published poster of study results is now available to review at: IntegroTheranostics.com/Science
Integro Theranostics will be initiating its Phase II lung cancer surgery study of CXL by the end of 2025.
Nicole Peterson
Integro Theranostics
+1 314-325-1800
npeterson@kingdomcapital.com
Visit us on social media:
LinkedIn
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.